多不饱和脂肪酸对健康儿童及成人认知功能的作用


总之,基础研究为LC omega-3 PUFA(尤其是DHA)在大脑功能中起重要作用的观点提供了强有力的支持;但是,食用LC omega-3多不饱和脂肪酸会提高健康学龄儿童和整个成年期的认知能力吗,特别是在饮食摄入量低的人群中?
DHA对学龄儿童认知发展的影响
DHA在孕晚期至2岁的大脑快速发育阶段尤为重要。之后,额叶在整个儿童期、青春期和二十多岁时继续发育,尤其在7-9岁和青春期中期发育迅速。表1总结了所有研究LC omega-3 PUFA对健康学龄儿童认知功能、学习和学业成绩影响的营养干预试验。Kuratko等人回顾了截至2012年11月发表的关于DHA与健康儿童学习和行为的研究。从2007年起,少量研究(n = 10)表明针对健康学龄儿童的临床试验证据较新。大多数研究在6-12岁儿童中进行,持续时间从8周到12个月不等,剂量从0.1到1.2克LC omega-3/天不等,干预措施的DHA:EPA比例和类型也有差异(鱼油、藻油、强化食品)。这些研究主要在LC omega-3 PUFA基线摄入量低的儿童中进行。麦克纳马拉等人首次在人类中展示了DHA与大脑激活的直接联系。在8-10岁男孩的持续注意力任务中,补充0.4克/天和1.2克/天的DHA可增加背外侧前额叶皮层的激活。但这些影响未转化为视觉持续注意力表现的改善,可能需要更长时间才能改善认知能力。

Richardson等人表明,补充DHA可以改善阅读表现不佳儿童的阅读能力。阅读分数在第20百分位及以下的儿童在补充DHA后阅读年龄额外增加了0.8个月,而在第10百分位及以下的儿童阅读年龄增加了1.9个月。Parletta等人在澳大利亚土著儿童中显示,EPA + DHA对认知发展(画人)的改善对7-12岁儿童影响更大,但未表现出学业成绩(阅读和拼写)的进步。这些结果应在影响英语读写能力的多种因素背景下解释,例如语言经验、家庭支持、社会经济地位(SES)和学校出勤率。仅补充Omega-3可能不足以克服这些因素。对南非营养不良儿童和墨西哥儿童的研究显示,补充LC omega-3可改善学习和认知能力。而印度和印度尼西亚的研究未发现任何影响,可能是因为剂量较小且主要使用ALA。鲍姆加特纳等人首次在缺铁儿童中进行LC omega-3试验,表明EPA + DHA对工作记忆有负面影响,并显示性别相互作用,缺铁男孩在DHA的长期记忆和检索方面表现更好,而女孩表现较差。这些研究表明,营养不良人群中进行omega-3补充试验的复杂性,其他因素和营养缺乏可能影响结果,而这些人群最有可能从补充剂中受益。
在英国,两项研究表明,摄入DHA对健康学龄儿童的认知表现和学习没有益处。肯尼迪等人的研究力量不足且持续时间短(8周)。在Kirby等人的研究中,DHA组和安慰剂组的颊细胞EPA和DHA均增加,但DHA组增加幅度更大。研究之间的不一致可能归因于年龄和性别的潜在调节作用。Parletta等人表明,处于大脑和认知发展不同阶段的儿童以及男孩和女孩对LC omega-3 PUFA补充剂的反应可能不同。在美国6-16岁儿童中,女孩饮食omega-3 PUFA与认知测试分数的关系是男孩的两倍。其他研究未调查年龄和性别的潜在交互作用,使用广泛年龄范围可能导致结局变异性或反应调节作用更大。

在儿童研究中,LC omega-3 PUFA摄入量的生物标志物通常不被测量,因为儿童害怕采集血液样本,因此不愿参加研究。有时会收集脸颊细胞样本,侵入性较小,并与饮食摄入量、血浆和红细胞水平密切相关。表1中的研究测量了红细胞、血浆、红细胞或血浆磷脂(PL)和脸颊细胞中的DHA和EPA水平/浓度。在所有研究中,补充LC omega-3 PUFA的水平均升高,增加幅度通常反映补充剂量。
迄今为止,尚未在青少年中进行LC omega-3 PUFA干预,唯一的证据来自观察性研究。一项前瞻性研究纳入了9000名>15岁瑞典学龄儿童,结果显示,每周食用鱼类超过1次的青少年在16岁时学习成绩显著高于每周食用鱼少于1次的青少年。在18岁时,每周吃鱼超过一次的男性青少年与15岁时每周吃鱼少于一次的男性青少年的智商分数更高,这是瑞典强制兵役征兵考试的一部分,通过完成的智力测试获得。De Groot等人最近在700名12-18岁荷兰青少年中表明,与不摄入鱼类相比,摄入提供推荐量的EPA + DHA为~0.45克/天的鱼与更高级的词汇和更高的期末成绩有关,但吃超过推荐量的鱼并没有更多益处。
总的来说,识字能力低和营养不良的儿童在认知结果(如记忆力、非语言认知发展、处理速度、视觉感知能力、注意力和执行功能)和学业成绩(如阅读和拼写)方面似乎可以从摄入LC omega-3 PUFA中受益最多。研究之间的不一致可能由于剂量、持续时间、其他营养素缺乏以及缺乏对性别和年龄交互作用的调查。在几项未显示益处的研究中,剂量可能太低。营养缺乏症(如营养不良人群的缺铁症)可能需要在开始补充LC omega-3脂肪酸之前进行纠正,以避免营养缺乏症与omega-3补充剂之间潜在的不良相互作用。
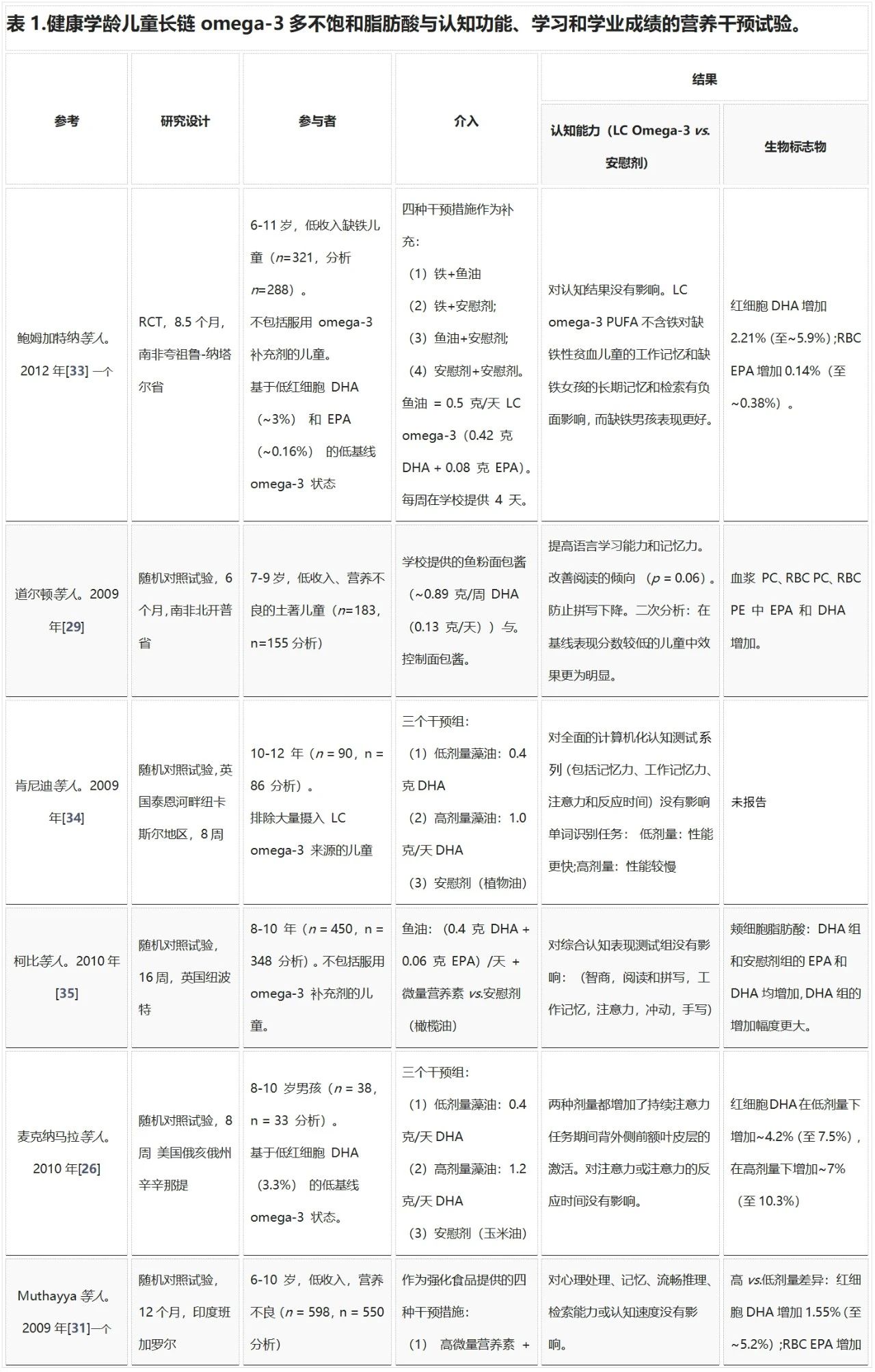
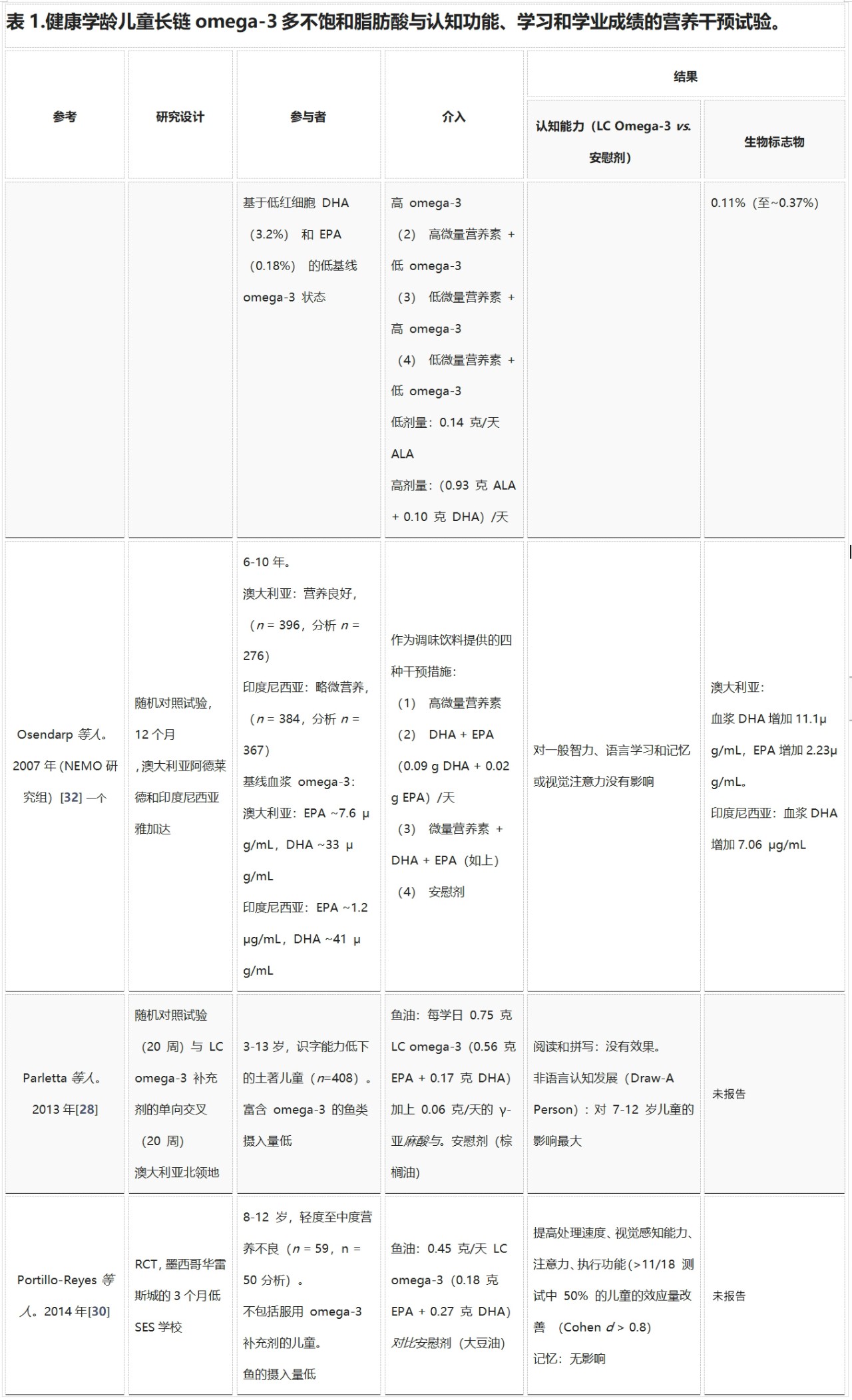

缩写:ALA,α-亚麻酸;DHA,二十二碳六烯酸;EPA,二十碳五烯酸;SES,社会经济地位;LC,长链;NR,未报告;PC,磷脂酰胆碱;PE,磷脂酰乙醇胺;红细胞,红细胞;RCT,随机对照试验;一个多个补充组,仅报告了 omega-3 研究组。
DHA对健康年轻人认知功能的影响
成年早期是保持大脑功能最佳状态的重要时期。尽管大脑发育已经完成,但神经可塑性仍在继续。目前只有七项研究探讨了长链omega-3多不饱和脂肪酸(LC omega-3 PUFA)对年轻健康成年人认知能力的影响(见表2)。其中,Stonehouse等人的研究是迄今为止规模最大、时间最长的试验之一,发现补充DHA可以改善记忆力和记忆反应时间。

Stonehouse等人的研究也是唯一一项探讨性别和载脂蛋白E基因型(APOE)是否会调节LC omega-3 PUFA补充剂反应的研究。结果显示,男性和女性在记忆领域受DHA影响不同;与安慰剂相比,女性的情景记忆得到改善,而男性的工作记忆反应时间有所改善。这种差异可能是由于男性和女性在执行记忆任务时使用了不同的问题解决策略,反映了大脑功能组织的差异。
虽然APOE对整个组的反应没有显著影响,但在按性别分层后,发现男性APOE4等位基因携带者在工作记忆和注意力反应时间方面的改善更为显著。载脂蛋白E是脑组织中的主要脂质转运蛋白,APOE4等位基因的变异携带者患阿尔茨海默病的风险显著增加(APOE3/E4和APOE4/E4的风险分别增加约3倍和15倍)。有趣的是,年轻(20-35岁)APOE4携带者在认知任务上的表现优于非携带者,这可能是因为他们在记忆任务期间大脑的额叶和颞叶区域激活增加。
表2中总结的其他随机对照试验均未显示LC omega-3 PUFA对认知有明显益处。例如,Fontani等人显示持续注意力和反应时间有所改善,但由于试验未报告安慰剂结果,因此需谨慎解读这些结果。其他研究中,样本量小、DHA剂量较低、参与者年龄范围广(18-70岁)以及试验持续时间较短(4-12周),这些因素可能影响了结果的可靠性。
综上所述,虽然部分研究显示补充DHA对认知功能有益,但性别、APOE基因型等因素可能会影响结果。因此,在未来的研究中,需要更大规模、更长时间的试验,并考虑性别和基因型的影响,以更全面地了解LC omega-3 PUFA对认知健康的潜在益处。
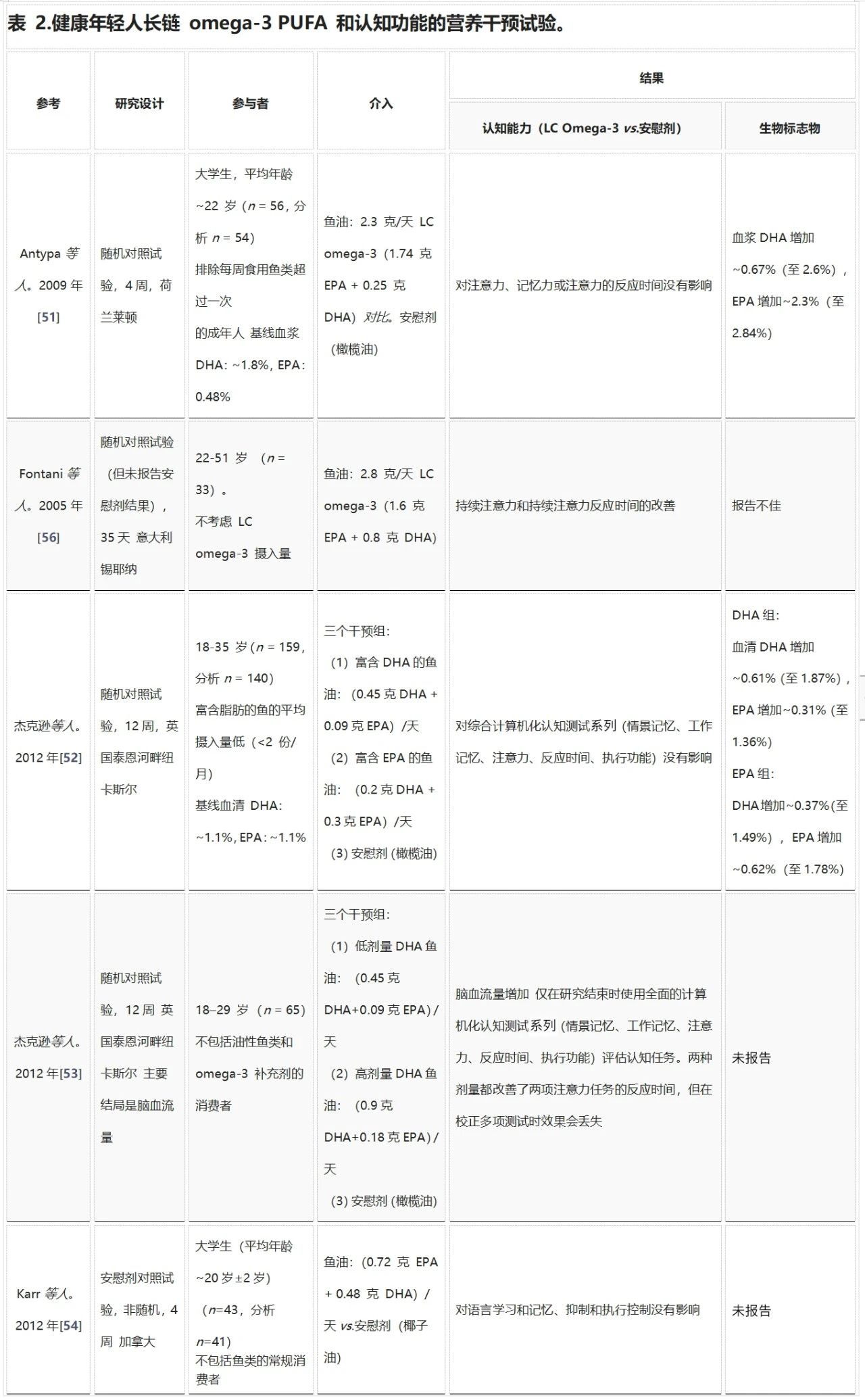
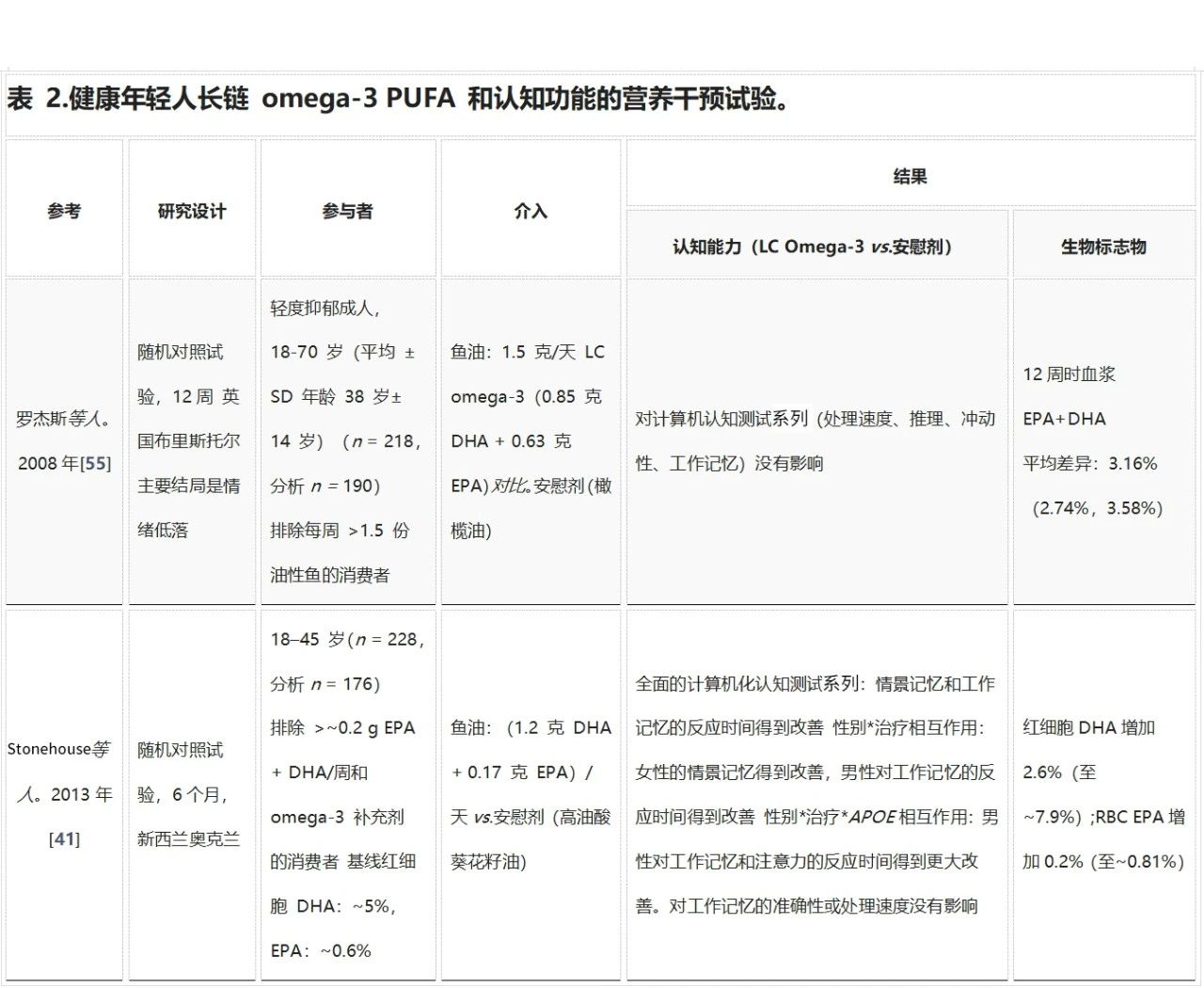
缩写:DHA,二十二碳六烯酸;EPA,二十碳五烯酸;APOE,载脂蛋白E基因型;LC, 长链;NR,未报告;红细胞,红细胞;RCT,随机对照试验;Narendran等人。[57] 未包含在表中,因为重点是机制性的,并且没有使用RCT研究设计。

补充LC omega-3 PUFA后,儿童的改善结果包括语言学习和记忆、阅读、拼写、非语言认知发展和处理速度、视觉感知能力、注意力和执行功能;在年轻人中,记忆力和记忆反应时间得到改善。
参考文献
1.Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar]
2.Chung, W.L.; Chen, J.J.; Su, H.M. Fish oil supplementation of control and (n-3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J. Nutr. 2008, 138, 1165–1171. [Google Scholar]
3.Gamoh, S.; Hashimoto, M.; Sugioka, K.; Hossain, M.S.; Hata, N.; Misawa, Y.; Masumura, S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience 1999, 93, 237–241. [Google Scholar] [CrossRef]
4.Horrocks, L.A.; Farooqui, A.A. Docosahexaenoic acid in the diet: Its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukotrienes Essent. Fatty Acids 2004, 70, 361–372. [Google Scholar] [CrossRef]
5.Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar]
6.Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
7.Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef]
8.Tassoni, D.; Kaur, G.; Weisinger, R.S.; Sinclair, A.J. The role of eicosanoids in the brain. Asia Pac. J. Clin. Nutr. 2008, 17, 220–228. [Google Scholar]
9.Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: A near IR spectroscopy pilot study. Br. J. Nutr. 2012, 107, 1093–1098. [Google Scholar] [CrossRef]
10.Cunnane, S.C.; Plourde, M.; Pifferi, F.; Begin, M.; Feart, C.; Barberger-Gateau, P. Fish, docosahexaenoic acid and Alzheimer’s disease. Progr. Lipid Res. 2009, 48, 239–256. [Google Schol
11.Rapoport, S.I. Translational studies on regulation of brain docosahexaenoic acid (DHA) metabolism in vivo. Prostaglandins Leukotrienes Essent. Fatty Acids 2013, 88, 79–85. [Google Scholar] [CrossRef]
12.Freemantle, E.; Vandal, M.; Tremblay-Mercier, J.; Tremblay, S.; Blachere, J.C.; Begin, M.E.; Brenna, J.T.; Windust, A.; Cunnane, S.C. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukotrienes Essent. Fatty Acids 2006, 75, 213–220. [Google Scholar] [CrossRef]
13.Umhau, J.C.; Zhou, W.; Carson, R.E.; Rapoport, S.I.; Polozova, A.; Demar, J.; Hussein, N.; Bhattacharjee, A.K.; Ma, K.; Esposito, G.; et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid Res. 2009, 50, 1259–1268. [Google Scholar] [CrossRef]
14.Moriguchi, T.; Salem, N., Jr. Recovery of brain docosahexaenoate leads to recovery of spatial task performance. J. Neurochem. 2003, 87, 297–309. [Google Scholar] [CrossRef]
15.Barcelo-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Progr. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
16.Sanders, T.A. DHA status of vegetarians. Prostaglandins Leukotrienes Essent. Fatty Acids 2009, 81, 137–141. [Google Scholar] [CrossRef]
17.Sarter, B.; Kelsey, K.S.; Schwartz, T.A.; Harris, W.S. Blood docosahexaenoic acid and eicosapentaenoic acid in vegans: Associations with age and gender and effects of an algal-derived omega-3 fatty acid supplement. Clin. Nutr. 2014, in press. [Google Scholar]
18.University of Otago; Ministry of Health. A Focus on Nutrition: Key findings of the 2008/2009 New Zealand Adult Nutrition Survey; Ministry of Health: Wellington, New Zealand, 2011. [Google Scholar]
19.Rahmawaty, S.; Charlton, K.; Lyons-Wall, P.; Meyer, B.J. Dietary intake and food sources of EPA, DPA and DHA in Australian children. Lipids 2013, 48, 869–877. [Google Scholar] [CrossRef]
20.Howe, P.; Meyer, B.; Record, S.; Baghurst, K. Dietary intake of long-chain omega-3 polyunsaturated fatty acids: Contribution of meat sources. Nutrition 2006, 22, 47–53. [Google Scholar] [CrossRef]
21.Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L., III. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef]
22.Sioen, I.; Vyncke, K.; de Maeyer, M.; Gerichhausen, M.; de Henauw, S. Dietary intake and food sources of total and individual polyunsaturated fatty acids in the Belgian population over 15 years old. Lipids 2013, 48, 729–738. [Google Scholar] [CrossRef]
23.Hughes, D.; Bryan, J. The assessment of cognitive performance in children: Considerations for detecting nutritional influences. Nutr. Rev. 2003, 61, 413–422. [Google Scholar] [CrossRef]
24.Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: A review of human studies. Prostaglandins Leukotrienes Essent. Fatty Acids 2010, 82, 305–314. [Google Scholar] [CrossRef]
25.Kuratko, C.N.; Barrett, E.C.; Nelson, E.B.; Salem, N., Jr. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: A review. Nutrients 2013, 5, 2777–2810. [Google Scholar] [CrossRef]
26.McNamara, R.K.; Able, J.; Jandacek, R.; Rider, T.; Tso, P.; Eliassen, J.C.; Alfieri, D.; Weber, W.; Jarvis, K.; DelBello, M.P.; et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: A placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 2010, 91, 1060–1067. [Google Scholar] [CrossRef]
27.Richardson, A.J.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Montgomery, P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: A randomized, controlled trial (the DOLAB Study). PLoS One 2012, 7, e43909. [Google Scholar]
28.Parletta, N.; Cooper, P.; Gent, D.N.; Petkov, J.; O’Dea, K. Effects of fish oil supplementation on learning and behaviour of children from Australian Indigenous remote community schools: A randomised controlled trial. Prostaglandins Leukotrienes Essent. Fatty Acids 2013, 89, 71–79. [Google Scholar] [CrossRef]
29.Dalton, A.; Wolmarans, P.; Witthuhn, R.C.; van Stuijvenberg, M.E.; Swanevelder, S.A.; Smuts, C.M. A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins Leukotrienes Essent. Fatty Acids 2009, 80, 143–149. [Google Scholar] [CrossRef]
30.Portillo-Reyes, V.; Perez-Garcia, M.; Loya-Mendez, Y.; Puente, A.E. Clinical significance of neuropsychological improvement after supplementation with omega-3 in 8–12 years old malnourished Mexican children: A randomized, double-blind, placebo and treatment clinical trial. Res. Dev. Disabil. 2014, 35, 861–870. [Google Scholar] [CrossRef]
31.Muthayya, S.; Eilander, A.; Transler, C.; Thomas, T.; van der Knaap, H.C.; Srinivasan, K.; van Klinken, B.J.; Osendarp, S.J.M.; Kurpad, A.V. Effect of fortification with multiple micronutrients and n-3 fatty acids on growth and cognitive performance in Indian schoolchildren: The CHAMPION (Children’s Health and Mental Performance Influenced by Optimal Nutrition) Study. Am. J. Clin. Nutr. 2009, 89, 1766–1775. [Google Scholar] [CrossRef]
32.Osendarp, S.J.; Baghurst, K.I.; Bryan, J.; Calvaresi, E.; Hughes, D.; Hussaini, M.; Karyadi, S.J.; van Klinken, B.J.; van der Knaap, H.C.; Lukito, W.; et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 Parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am. J. Clin. Nutr. 2007, 86, 1082–1093. [Google Scholar]
33.Baumgartner, J.; Smuts, C.M.; Malan, L.; Kvalsvig, J.; van Stuijvenberg, M.E.; Hurrell, R.F.; Zimmermann, M.B. Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: A randomized, double-blind, placebo-controlled intervention in South Africa. Am. J. Clin. Nutr. 2012, 96, 1327–1338. [Google Scholar] [CrossRef]
34.Kennedy, D.O.; Jackson, P.A.; Elliott, J.M.; Scholey, A.B.; Robertson, B.C.; Greer, J.; Tiplady, B.; Buchanan, T.; Haskell, C.F. Cognitive and mood effects of 8 weeks’ supplementation with 400 mg or 1000 mg of the omega-3 essential fatty acid docosahexaenoic acid (DHA) in healthy children aged 10–12 years. Nutr. Neurosci. 2009, 12, 48–56. [Google Scholar] [CrossRef]
35.Kirby, A.; Woodward, A.; Jackson, S.; Wang, Y.; Crawford, M.A. A double-blind, placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Res. Dev. Disabil. 2010, 31, 718–730. [Google Scholar] [CrossRef]
36.Lassek, W.D.; Gaulin, S.J. Sex differences in the relationship of dietary Fatty acids to cognitive measures in american children. Front. Evol. Neurosci. 2011, 3, 5. [Google Scholar]
37.Connor, S.L.; Zhu, N.; Anderson, G.J.; Hamill, D.; Jaffe, E.; Carlson, J.; Connor, W.E. Cheek cell phospholipids in human infants: A marker of docosahexaenoic and arachidonic acids in the diet, plasma, and red blood cells. Am. J. Clin. Nutr. 2000, 71, 21–27. [Google Scholar]
38.Kim, J.L.; Winkvist, A.; Aberg, M.A.; Aberg, N.; Sundberg, R.; Toren, K.; Brisman, J. Fish consumption and school grades in Swedish adolescents: A study of the large general population. Acta Paediatr. 2010, 99, 72–77. [Google Scholar]
39.Aberg, M.A.; Aberg, N.; Brisman, J.; Sundberg, R.; Winkvist, A.; Toren, K. Fish intake of Swedish male adolescents is a predictor of cognitive performance. Acta Paediatr. 2009, 98, 555–560. [Google Scholar] [CrossRef]
40.De Groot, R.H.; Ouwehand, C.; Jolles, J. Eating the right amount of fish: Inverted U-shape association between fish consumption and cognitive performance and academic achievement in Dutch adolescents. Prostaglandins Leukotrienes Essent. Fatty Acids 2012, 86, 113–117. [Google Scholar] [CrossRef] [Green Version]
41.Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef]
42.Guillem, F.; Mograss, M. Gender differences in memory processing: Evidence from event-related potentials to faces. Brain Cogn. 2005, 57, 84–92. [Google Scholar] [CrossRef]
43.Speck, O.; Ernst, T.; Braun, J.; Koch, C.; Miller, E.; Chang, L. Gender differences in the functional organization of the brain for working memory. Neuroreport 2000, 11, 2581–2585. [Google Scholar] [CrossRef]
44.Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef]
45.Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease—A meta-analysis. J. Am. Med. Assoc. 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
46.Dennis, N.A.; Browndyke, J.N.; Stokes, J.; Need, A.; Buke, J.R.; Welsh-Bohmer, K.A.; Cabeza, R. Temporal lobe functional activity and connectivity in young adult APOE epsilon 4 carriers. Alzheimer’s Dement. 2010, 6, 303–311. [Google Scholar] [CrossRef]
47.Filippini, N.; Ebmeier, K.P.; MacIntosh, B.J.; Trachtenberg, A.J.; Frisoni, G.B.; Wilcock, G.K.; Beckmann, C.M.; Smith, S.M.; Matthews, P.M.; Mackay, C.E. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage 2011, 54, 602–610. [Google Scholar] [CrossRef]
48.Filippini, N.; MacIntosh, B.J.; Hough, M.G.; Goodwin, G.M.; Frisoni, G.B.; Smith, S.M.; Matthews, P.M.; Beckmann, C.F.; Mackay, C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon 4 allele. Proc. Natl. Acad. Sci. USA 2009, 106, 7209–7214. [Google Scholar] [CrossRef]
49.Alexander, D.M.; Williams, L.M.; Gatt, J.M.; Dobson-Stone, C.; Kuan, S.A.; Todd, E.G.; Schofield, P.R.; Cooper, N.J.; Gordon, E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol. Psychol. 2007, 75, 229–238. [Google Scholar] [CrossRef]
50.Mondadori, C.R.A.; de Quervain, D.J.-F.; Buchmann, A.; Mustovic, H.; Wollmer, M.A.; Schmidt, C.F.; Boesiger, P.; Christoph Hock, C.; Nitsch1, R.M.; Papassotiropoulos1, A.; et al. Better memory and neural efficiency in young apolipoprotein E epsilon 4 carriers. Cerebr. Cortex 2007, 17, 1934–1947. [Google Scholar] [CrossRef]
51.Antypa, N.; van der Does, A.J.W.; Smelt, A.H.M.; Rogers, R.D. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J. Psychopharmacol. 2009, 23, 831–840. [Google Scholar] [CrossRef]
52.Jackson, P.A.; Deary, M.E.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–35 years. Br. J. Nutr. 2012, 107, 1232–1243. [Google Scholar] [CrossRef]
53.Jackson, P.A.; Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Docosahexaenoic acid-rich fish oil modulates the cerebral hemodynamic response to cognitive tasks in healthy young adults. Biol. Psychol. 2012, 89, 183–190. [Google Scholar] [CrossRef]
54.Karr, J.E.; Grindstaff, T.R.; Alexander, J.E. Omega-3 polyunsaturated fatty acids and cognition in a college-aged population. Exp. Clin. Psychopharmacol. 2012, 20, 236–242. [Google Scholar] [CrossRef]
55.Rogers, P.J.; Appleton, K.M.; Kessler, D.; Peters, T.J.; Gunnell, D.; Hayward, R.C.; Heatherley, S.V.; Christian, L.M.; McNaughton, S.A.; Ness, A.R. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: A randomised controlled trial. Br. J. Nutr. 2008, 99, 421–431. [Google Scholar]
56.Fontani, G.; Corradeschi, F.; Felici, A.; Alfatti, F.; Migliorini, S.; Lodi, L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur. J. Clin. Investig. 2005, 35, 691–699. [Google Scholar] [CrossRef]
57.Narendran, R.; Frankle, W.G.; Mason, N.S.; Muldoon, M.F.; Moghaddam, B. Improved working memory but no effect on striatal vesicular monoamine transporter type 2 after omega-3 polyunsaturated fatty acid supplementation. PLoS One 2012, 7, e46832. [Google Scholar]
58.Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N., Jr.; Stedman, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer’s Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]
59.Lee, L.K.; Shahar, S.; Chin, A.V.; Yusoff, N.A. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): A 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology 2013, 225, 605–612. [Google Scholar] [CrossRef]
60.Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R.C. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef]
61.Dangour, A.D.; Allen, E.; Elbourne, D.; Fasey, N.; Fletcher, A.E.; Hardy, P.; Holder, G.E.; Knight, R.; Letley, L.; Richards, M.; et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: A randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 2010, 91, 1725–1732. [Google Scholar] [CrossRef]
62.Geleijnse, J.M.; Giltay, E.J.; Kromhout, D. Effects of n-3 fatty acids on cognitive decline: A randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients. Alzheimer’s Dement. 2012, 8, 278–287. [Google Scholar] [CrossRef]
63.Stough, C.; Downey, L.; Silber, B.; Lloyd, J.; Kure, C.; Wesnes, K.; Camfield, D. The effects of 90-day supplementation with the omega-3 essential fatty acid docosahexaenoic acid (DHA) on cognitive function and visual acuity in a healthy aging population. Neurobiol. Aging 2012, 33, 824.e1–824.e3. [Google Scholar]
64.Van de Rest, O.; Geleijnse, J.M.; Kok, F.J.; van Staveren, W.A.; Dullemeijer, C.; OldeRikkert, M.G.M.; Beekman, A.T.F.; de Groot, C.P.G.M. Effect of fish oil on cognitive performance in older subjectse—A randomized, controlled trial. Neurology 2008, 71, 430–438. [Google Scholar] [CrossRef]
65.Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef]
66.Nilsson, A.; Radeborg, K.; Salo, I.; Bjorck, I. Effects of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: A randomized controlled cross-over study. Nutr. J. 2012, 11, 99. [Google Scholar] [CrossRef]
67.Vakhapova, V.; Cohen, T.; Richter, Y.; Herzog, Y.; Korczyn, A.D. Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: A double-blind placebo-controlled trial. Dement. Geriatr. Cogn. Disord. 2010, 29, 467–474. [Google Scholar] [CrossRef]
68.Witte, A.V.; Kerti, L.; Hermannstadter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-Chain Omega-3 Fatty Acids Improve Brain Function and Structure in Older Adults. Cerebr. Cortex 2013. [Google Scholar] [CrossRef]
69.Konagai, C.; Yanagimoto, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Koga, Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clin. Interv. Aging 2013, 8, 1247–1257. [Google Scholar]
70.Richter, Y.; Herzog, Y.; Lifshitz, Y.; Hayun, R.; Zchut, S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: A pilot study. Clin. Interv. Aging 2013, 8, 557–563. [Google Scholar]
71.Salem, N., Jr.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef]
72.Milte, C.M.; Sinn, N.; Howe, P.R. Polyunsaturated fatty acid status in attention deficit hyperactivity disorder, depression, and Alzheimer’s disease: Towards an omega-3 index for mental health? Nut. Rev. 2009, 67, 573–590. [Google Scholar] [CrossRef]
73.Harris, W.S.; von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
74.Kuratko, C.N.; Salem, N. Biomarkers of DHA status. Prostaglandins Leukotrienes Essent. Fatty Acids 2009, 81, 111–118. [Google Scholar] [CrossRef]
75.Lapillonne, A.; deMar, J.C.; Nannegari, V.; Heird, W.C. The fatty acid profile of buccal cheek cell phospholipids is a noninvasive marker of long-chain polyunsaturated fatty acid status in piglets. J. Nutr. 2002, 132, 2319–2323. [Google Scholar]
76.Bell, J.G.; Mackinlay, E.E.; Dick, J.R.; Younger, I.; Lands, B.; Gilhooly, T. Using a fingertip whole blood sample for rapid fatty acid measurement: Method validation and correlation with erythrocyte polar lipid compositions in UK subjects. Br. J. Nutr. 2011, 106, 1408–1415. [Google Scholar] [CrossRef]
77.Harris, W.S. The omega-3 index: Clinical utility for therapeutic intervention. Curr. Cardiol. Rep. 2010, 12, 503–508. [Google Scholar] [CrossRef]
78.Katan, M.B.; Deslypere, J.P.; van Birgelen, A.P.; Penders, M.; Zegwaard, M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. J. Lipid Res. 1997, 38, 2012–2022. [Google Scholar]
79.Flock, M.R.; Skulas-Ray, A.C.; Harris, W.S.; Etherton, T.D.; Fleming, J.A.; Kris-Etherton, P.M. Determinants of Erythrocyte Omega-3 Fatty Acid Content in Response to Fish Oil Supplementation: A Dose-Response Randomized Controlled Trial. J. Am. Heart Assoc. 2013, 2. [Google Scholar] [CrossRef]
80.MacIntosh, B.A.; Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Mangan, M.; Hibbeln, J.R.; Mann, J.D. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br. J. Nutr. 2013, 110, 559–568. [Google Scholar] [CrossRef]
81.Moriguchi, T.; Loewke, J.; Garrison, M.; Catalan, J.N.; Salem, N., Jr. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J. Lipid Res. 2001, 42, 419–427. [Google Scholar]
82.Connor, W.E.; Neuringer, M.; Lin, D.S. Dietary effects on brain fatty acid composition: The reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J. Lipid Res. 1990, 31, 237–247. [Google Scholar]
83.Kennedy, D.O. Reply to MP Pase and C Stough. Am. J. Clin. Nutr. 2013, 98, 510–511. [Google Scholar] [CrossRef]
84.Schmitt, J.A.; Benton, D.; Kallus, K.W. General methodological considerations for the assessment of nutritional influences on human cognitive functions. Eur. J. Nutr. 2005, 44, 459–464. [Google Scholar] [CrossRef]
85.Dangour, A.D.; Allen, E. Do omega-3 fats boost brain function in adults? Are we any closer to an answer? Am. J. Clin. Nutr. 2013, 97, 909–910. [Google Scholar] [CrossRef]
86.Sizonenko, S.V.; Babiloni, C.; Sijben, J.W.; Walhovd, K.B. Brain imaging and human nutrition: Which measures to use in intervention studies? Adv. Nutr. 2013, 4, 554–556. [Google Scholar] [CrossRef]
87.Geppert, J.; Kraft, V.; Demmelmair, H.; Koletzko, B. Docosahexaenoic acid supplementation in vegetarians effectively increases omega-3 index: A randomized trial. Lipids 2005, 40, 807–814. [Google Scholar] [CrossRef]
88.Flock, M.R.; Harris, W.S.; Kris-Etherton, P.M. Long-chain omega-3 fatty acids: Time to establish a dietary reference intake. Nutr. Rev. 2013, 71, 692–707. [Google Scholar] [CrossRef]
89.Australian National Health and Medical Research Council; New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand; Commonwealth of Australia: Canberra, Australia, 2006. [Google Scholar]
90.Meyer, B.J.; Kolanu, N. Australian children are not consuming enough long-chain omega-3 polyunsaturated fatty acids for optimal health. Nutrition 2011, 27, 1136–1140. [Google Scholar] [CrossRef]


